Wound healing modulation by novel antimicrobial Peptides
- Biology
Corneal abrasion (the process of wearing something away) is the most common eye injury. A common complication of corneal abrasion is corneal infection (keratitis). The National Eye Institute reports approximately 30,000 cases of keratitis in the US every year. Of this number bacterial keratitis is prevalent. Bacterial keratitis is usually caused by infectious microorganisms Staphylococcus aureus and Pseudomonas aeruginosa. Infections caused by P. aeruginosa are particularly aggressive and can leave an individual blind within 36 hours. The cornea itself is a highly complex structure forming the outermost layer of the eye. This structure plays an important role in refracting light to the back of the eye for visual processing, mechanically protecting the eye against dirt, bacteria and other matter that can harm the eye and the individual. Slight injuries to the cornea can usually heal naturally, but underlying conditions such as diabetes or dry eye can complicate this natural process. Deeper injuries can lead to permanent tissue scarring and vision loss. The cornea is the first barrier of defence against anything from the outside world. If the cornea becomes damaged, the second line of defence is the response of the body’s innate immune system. This system is comprised of specialised cells that act like the body’s own in-house paramedics; rushing in at the site of injury providing protection and healing to the affected area. Dr Kasus-Jacobi and her research team work on elucidating wound healing mechanisms associated with cells of the innate immune system and the development of novel therapies with antimicrobial and healing properties.
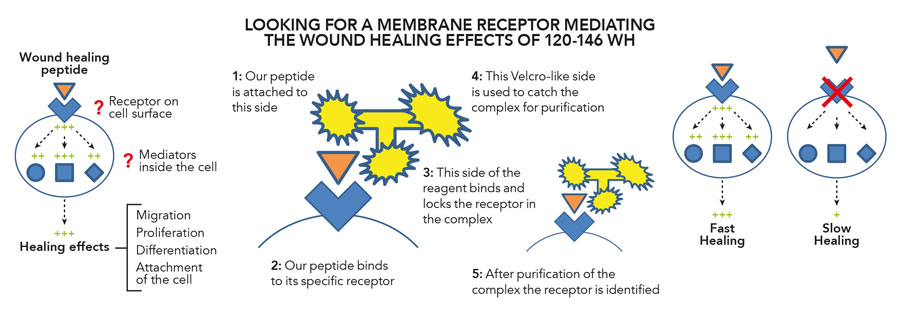
Figure 2: Innovative tool to identify candidate receptors of our wound healing peptide.
Figure 3: Confirming the identity of the receptor.
A unique antimicrobial protein
The most anterior layer of the cornea is comprised of the epithelium; this is a film of cells comprised of an outer, middle and inner layer. When the corneal epithelium is damaged, various wound-healing mechanisms are activated. They involve the employment of proteins that promote cell proliferation, migration and repair. In previous studies, the antimicrobial protein CAP37 was identified in granules in immune cells that are known to swarm to the site of the damaged cornea called neutrophils. Neutrophils are a part of the human body’s innate immune system and are usually the first type of immune cell to arrive at an area of damaged tissue. This cell is characterised by its ability to mediate acute inflammatory responses and remove infectious microorganisms and aged or damaged cells. In a previous study by Dr Pereira, not only was CAP37 found within circulating neutrophils as expected, but also within corneal epithelial cells that constitute the epithelium of the cornea. This finding suggested that CAP37 may serve as an important regulator of inflammation and corneal wound healing. In a more recent study, Drs Pereira, Griffith and Kasus-Jacobi investigated the corneal wound healing ability of CAP37 via the use of a mouse model. Damaged mouse corneal epithelial cells treated with CAP37 showed accelerated wound closure and healing. Further analysis inferred that CAP37 affected corneal epithelial wound healing primarily by epithelial cell migration in the early stages of wound repair. Additionally, CAP37 treatment of wounded mouse corneal epithelial cells showed an increased presence of protein kinase C delta (PKCδ). Protein kinases are enzymes that modulate the biological activity of other proteins within the body. In a previous study, it was found that PKCδ promoted CAP37-induced chemotaxis of corneal epithelial cells. Chemotaxis is the movement of a cell or organism in the direction of a chemical stimulus. This study was the first to consolidate CAP37-induced corneal epithelial wound healing through the protein kinase C signalling pathway, particularly by PKCδ. Further delineation of the role of CAP37 in corneal epithelial cell with regard to cellular and molecular processes facilitating wound healing, will enable the design of novel therapeutics for corneal wound healing and infections.
This study was the first to consolidate CAP37-induced corneal epithelial wound healing through the protein kinase C signalling pathway, particularly by PKCδ.
Development of novel therapeutics
Jacobi, small fragments of CAP37 were used to identify domains or areas relevant to its antimicrobial activity in order to synthesise bioactive peptides for use as novel therapies for corneal abrasion. Like proteins, peptides are functionally important in a complex cellular process involving cell-cell signalling, maintaining cell structure and regulation. Both proteins and peptides are made of the same molecular building blocks called amino acids. Synthesis of bioactive peptides effectively means recreating a part of the structure of a desired protein like CAP37 that has a known unique property; modifying the amino acid structure almost like recombining pieces of Lego to optimise its structure and function. Using this type of modification, a collection of peptides was generated and Dr Kasus-Jacobi and her team of researchers were able to identify a subset of peptide derived from CAP37 with increased bactericidal activity. One of these synthesised peptides, 120-146 WH, was able to promote corneal epithelial wound healing and effective clearing of bacterial invader P. aeruginosa. Dr Kasus-Jacobi has since led research in demonstrating that further modifications of 120-146 WH could retain wound healing ability and increase killing efficiency of P. aeruginosa. Dr Kasus-Jacobi and research collaborators are pioneering the creation of such novel therapeutics for specialised ophthalmic treatment. The clinical development of such peptides could be an immense benefit, as the unique mechanism by which they function could help circumvent development of microbial antibiotic resistance developed against classical antibiotics.
Innovative receptor detection
In recent studies, Dr Kasus-Jacobi along with her research collaborators discovered that CAP37 binds to a specific receptor found on the outer surface membrane of human corneal epithelial cells (HCECs). Dr Kasus-Jacobi used an emerging powerful method of receptor capture and identification using a specialised reagent called TriCEPs, available through Dualsystems Biotech AG (https://www.dualsystems.com). Her research objectives encompassed the delineation of specific HCEC receptors binding CAP37, peptide 120-146 WH and a modified peptide 120-146 WH 5RMP. This process of ligand-receptor capture is like the analogy of a lock and key. The key is the ligand, a molecule or protein such as CAP37 and the lock is the cell surface membrane receptor that the ligand binds to. This ligand-receptor lock and key method of protein-cell interaction is the fundamental basis of how cells communicate and transduce signals for induction of cellular processes like CAP37-induced corneal epithelial wound healing. The TriCEPs reagent has three primary functionalities; one for binding the ligand of interest (here CAP37, or peptide), another for irreversible binding of cell surface receptors and one that serves as a handle for purification of the complex containing the unknown receptor. In the latest collaborative study led by Dr Kasus-Jacobi, CAP37 along with synthesised peptides were coupled with TriCEPs to identify their target receptors. The degree of coupling was tested and proteins analysed using a technique called mass spectrometry, which identifies specific proteins by using their mass. It was found that CAP37 and its derived peptides interacted with partner protein ligands S100A8 and S100A9. This finding greatly narrows down the potential membrane receptors used by CAP37 and derived peptides to promote corneal wound healing. Dr Kasus-Jacobi’s current research is directed at investigating the functional effect(s) of these interactions in relation to corneal epithelial wound healing.
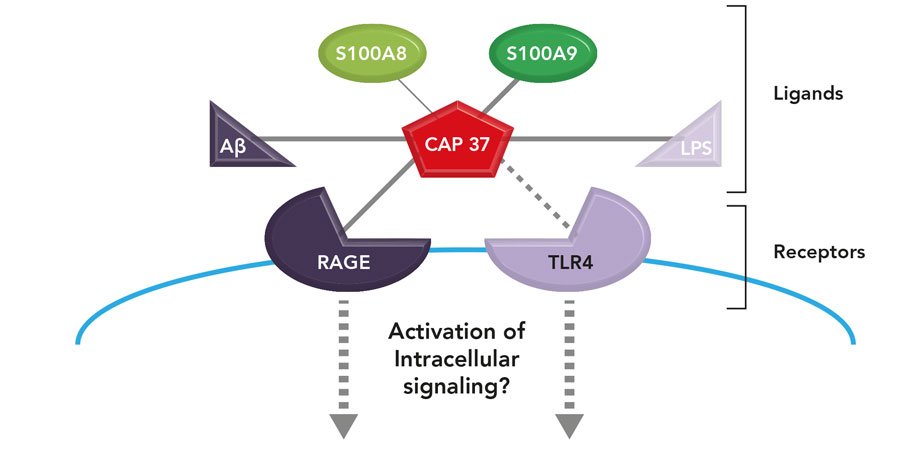
An emerging concept in our lab is that CAP37 (the key) could modulate the activation of RAGE and TLR4 receptors (the locks) by two mechanisms: a direct interaction with these receptors and an interaction with other ligands (or keys) for these receptors.
Dr Kasus-Jacobi’s pioneering research will enable the creation of novel therapeutic antimicrobial peptides to combat bacterial infection.
Future impact
Dr Kasus-Jacobi’s pioneering research in the field of corneal epithelial wound healing will enable the creation of novel therapeutic antimicrobial peptides to combat bacterial infection. Her research into the complex signalling components of wound healing will also provide invaluable insights into how the process is enacted by the body and its innate cellular mechanics. Not only are corneal injuries occurring within the bounds of home in all areas of the world; there are a substantial number of military personnel and civilians in war-stricken environments that can develop serious visual impairment from ineffective wound healing of worn corneal tissue. Dr Kasus-Jacobi’s work in this field provides the scientific community with a well-stocked armoury, from which, corneal injuries and associated bacterial infections can be actioned against to mitigate detrimental harm to individual health and well-being.
It was my encounter with Dr Pereira who pioneered the structure-function research on CAP37. She was looking for someone to lead the development of antimicrobial peptides derived from CAP37 into therapeutics for ocular injury. My background and interest in Ophthalmology, combined with the fact that corneal abrasion is the most common eye injury with a need for better ocular products led me to embark in this line of research. It has been gratifying to identify a unique peptide that fulfils two important functions: killing invading bacteria while promoting corneal wound healing at the same time. I believe a better understanding of these mechanisms will promote the development of innovative ocular products.
References
- Frei A.P, Jeon OY, et al. (2012). ‘Direct identification of ligand-receptor interactions on living cells and tissues’. Nature Biotech, vol. 30, no. 10, pp.997-1001.
- Frei A.P, Moest H, Novy K and Wollscheid B. (2013). ‘Ligand-based receptor identification on living cells and tissues using TRICEPS’. Nature Protocols, vol.8, no.7, pp.1321-1336.
- Griffith G.L, Kasus-Jacobi A, Lerner M R, Pereira H A. (2014). ‘Corneal Wound Healing, a Newly Identified Function of CAP37, is Mediated by Protein Kinase C Delta (PKCdelta)’. Investigative ophthalmology & visual science, vol.5, no.8, pp. 4886-4895. doi:10.1167/iovs.14-14461.
- Griffith G.L, Kasus-Jacobi A, Pereira H.A. (2017). ‘Bioactive Antimicrobial Peptides as Therapeutics for Corneal Wounds and Infections’. Advances in Wound Care, vol.6, no.6, pp.175-190. doi:10.1089/wound.2016.0713.
- Kasus-Jacobi A, Noor-Mohammadi S, Griffith G.L, Hinsley H, Mathias L, Pereira H.A. (2015). ‘A multifunctional peptide based on the neutrophil immune defense molecule, CAP37, has antibacterial and wound-healing properties’. Journal of Leukocyte Biology, vol. 97, pp. 341-50. DOI: 10.1189/jlb.3A0214-104RR.
The research project led by Dr Kasus-Jacobi aims to have a beneficial impact on the treatment of corneal abrasion and the prevention or treatment of vision-threatening bacterial keratitis.
Funding
- NIH National Eye Institute
- Oklahoma Center for the Advancement of Science and Technology
- Seed grant, College of Pharmacy, OU Health Sciences Center
Collaborators
- H. Anne Pereira, PhD, OU Health Sciences Center
- Gina L. Griffith, PhD, US Army Institute of Surgical Research
- Samaneh Noor-Mohammadi, PhD, Immuno-Mycologics, Inc.
- Amanda J. Stock, PhD, NIH National Institute of Aging
- Paul Helbling, PhD, Dualsystems Biotech AG
Bio
 Dr Kasus-Jacobi received her PhD from Paris IX University in Endocrinology. She then completed a postdoctoral fellowship at UT Southwestern Medical Center in Molecular Genetics. She went on to an independent research position at OU Health Sciences Center, first in the Department of Ophthalmology and currently in Pharmaceutical Sciences at the College of Pharmacy.
Dr Kasus-Jacobi received her PhD from Paris IX University in Endocrinology. She then completed a postdoctoral fellowship at UT Southwestern Medical Center in Molecular Genetics. She went on to an independent research position at OU Health Sciences Center, first in the Department of Ophthalmology and currently in Pharmaceutical Sciences at the College of Pharmacy.
Contact
Anne Kasus-Jacobi, PhD
Assistant Professor of Research
College of Pharmacy
Department of Pharmaceutical Sciences, CPB 318
University of Oklahoma Health Sciences Center
1110 North Stonewall Ave., Oklahoma City, OK 73117
USA
E: [email protected]
T: +1 405 637 3432
W: https://pharmacy.ouhsc.edu/directory/anne-kasus-jacobi-ph-d
W: http://profiles.ouhsc.edu/display/72509
W: www.researchgate.net/profile/Anne_Kasus-Jacobi
W: www.linkedin.com/in/anne-kasus-jacobi-1aa81976/
Creative Commons Licence
(CC BY-NC-ND 4.0) This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. Creative Commons License
What does this mean?
Share: You can copy and redistribute the material in any medium or format


Lost for words: investigating specific language impairments

A man who makes chromosomes

WormBot: Automating ageing experiments on C. elegans


Harnessing high-dimensional data in environmental health sciences




