Characterising tertiary lymphoid structures in lupus-prone mice
- Health & Medicine
Tertiary lymphoid structures can form in the kidneys of people with systemic lupus erythematosus (SLE). Structurally, they resemble lymph nodes and are formed in non-lymphoid tissues in response to chronic inflammation. Dr Kristin Fenton and Dr Hege Lynum Pedersen, based at The Arctic University of Norway, are exploring the cells and structures of these lymphoid aggregates to expand our knowledge of inflammatory processes in the kidneys of people with SLE and to pave the way for new diagnostic and treatment pathways.
Systemic lupus erythematosus (SLE) is an autoimmune disease, often characterised by pain and inflammation in the joints, lungs, heart, liver and kidneys. People with lupus often experience flare ups (relapses) alternating with no symptoms (remission); however, some people will experience chronic symptoms. Treatment often focuses on suppressing the immune response against the body’s cells, but this also makes people more susceptible to other infections as their immune system is weakened.
SLE occurs when antibodies bind to molecules, such as chromatin, proteins and DNA. The antibody-target complexes are then deposited in, or bind to, different tissues in the body. This is the reason that people with SLE can have a range of symptoms in different areas of their body. For example, when these immune complexes bind within the kidney of an individual with SLE, inflammation occurs, and lupus nephritis (LN) can develop. LN is a kidney disease that, over time, might lead to kidney failure.
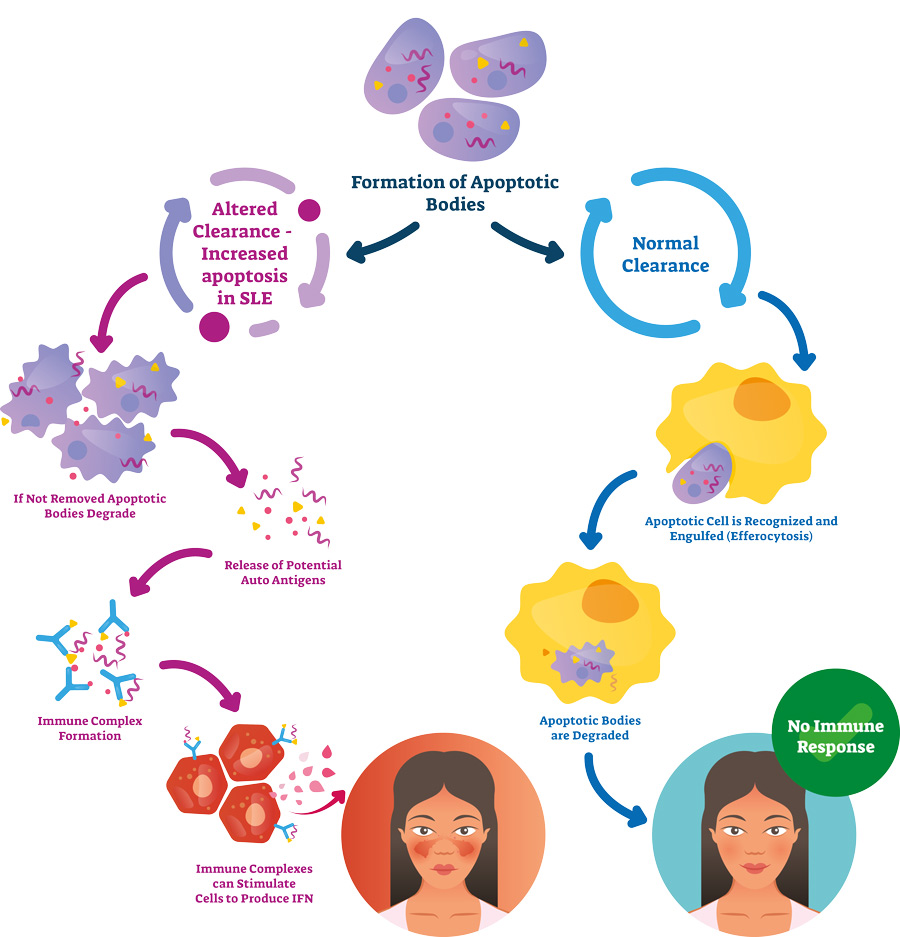
The immune complexes can bind within glomeruli, the filtration units of the kidney, or within the spaces between the tubuli, the tubes responsible for reabsorption and excretion processes within the kidney. Binding at either location initiates an immune response where signalling molecules called chemokines attract immune cells and activate intrinsic immune cells in the kidney. As a result of this inflammatory process, tertiary lymphoid structures (TLS) may form. TLS are immune aggregates containing a variety of cells that form at sites of infection or chronic inflammation. Currently, TLS are often not seen in renal biopsies since they only represent a very small area of the kidney.
“TLS are immune aggregates containing a variety of cells that form at sites of infection or chronic inflammation.”
Dr Kristin Fenton and Dr Hege Lynum Pedersen work in the Department of Medical Biology at The Arctic University of Norway. Through their research, the team aims to learn more about TLS in kidneys – particularly whether they are sites for activation or inhibition of immune cells.
Characterising tertiary lymphoid structures
Dr Fenton and Dr Pedersen investigated the formation of TLS in mice that spontaneously develop SLE and LN. They aimed to compare kidney-specific TLS that had been dissected from lupus-prone mice, with whole kidney (containing no TLS) and lymph nodes from the same mice. Lymph nodes are well known for being a secondary lymphoid organ. They play an important role in the immune system where they act as filters, identifying any foreign cells that pass through them in lymph fluid. This contrasts with primary lymphoid organs which produce and mature immune cells, such as T cells and B cells.
Using lupus-prone mice, the research group monitored the amount of protein in their urine to measure the levels of disease in the mouse kidneys. They also used blood samples to test for autoantibodies against DNA, the cause of LN and associated with TLS formation.
The research group then analysed the different cells that made up TLS, as well as looking at the cells associated with vessel formation and the localised area around the TLS in the kidney. They did this by using techniques called immunohistochemistry and immunofluorescence. Both methods are based on a similar principle. Immunohistochemistry uses fixed tissue sections embedded in paraffin, and immunofluorescence uses frozen tissue sections fixed on a slide. Primary antibodies are used to bind to the molecule of interest, for example, a cell surface receptor only found on immune B cells. Secondary antibodies, with a fluorescent tag in the case of immunofluorescence, then bind to the primary antibody. Specialised microscopes can be used to identify the cells of interest as they will have the specific antibodies bound to them.

PAS staining method.
They also analysed gene expression within TLS and compared it to gene expression in other tissues, such as lymph node, kidney tissue or whole kidney. Next-generation gene sequencing was used for this step, a process which allows a DNA sequence to be obtained by using a library of fragmented RNA sequences and re-assembling overlapping reads.
TLS have defined organisational and gene patterns
The researchers found that TLS were formed in mice that produced autoantibodies against DNA. All mice which had these antibodies for 5-6 weeks developed TLS. The structures tended to occur along the larger blood vessels of the kidney. Three-dimensional imaging showed that there were large, interconnected networks of immune aggregates close to the blood vessels.
The TLS also had defined organisational patterns. Specific zones containing different immune cells could be identified, for example distinct T cell areas surrounded by a B cell zone. Other immune cells, such as dendritic cells and macrophages, could also be identified. The team also found functional germinal centres, areas where mature B cells proliferate and differentiate into their different populations.

Gene expression in kidney TLS was similar to gene expression in lymph nodes, with 600 genes common to both. These included genes known to be involved in activation and regulation of the immune system.
The researchers compared gene expression in the mouse model of SLE with human LN gene expression. Interestingly, they were able to show similar patterns in gene expression where up-regulated genes reflected TLS formation and down-regulated genes were involved in metabolic processes of the kidney cells. For example, the results showed that genes Ltbr, Ccr7, Pouf2af1, Vcam1, Icam1 and Glycam were significantly upregulated in mice with late-stage lupus and their expression was positively correlated with autoantibody production and TLS formation.
“Dr Fenton and Dr Pedersen concluded that kidney TLS may function as a
kidney-specific type of lymph node.”
Mesenchymal stem cells
Other work done by the group at The Arctic University of Norway has investigated the presence of mesenchymal stem cells (MSC), cells which have the potential to differentiate into a variety of other cell types, in TLS. They also explored the role of MSCs in the activation of T cells using tissue samples from healthy individuals or those with SLE or LN. The data produced by Dr Fenton and Dr Pedersen suggest that MSC may have important role in the organisation of lymphoid tissue and may accelerate early inflammatory processes and initiate the formation of TLS in kidneys of individuals with chronic inflammatory conditions.

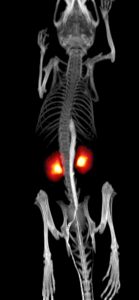
In vivo imaging to monitor disease
Dr Fenton and Dr Pedersen concluded that kidney TLS may function as a kidney-specific type of lymph node, as they both have a similar cell composition, structure and gene signature. Furthermore, changes in genes in the mouse kidneys appeared similar to the published gene expression profiles of kidney biopsies taken from humans with SLE and LN.
As the formation of TLS happens early in the disease process, it could therefore be a good target for early diagnosis and intervention. This would require the use of specialised in vivo imaging techniques to identify TLS, such as positron emission tomography (PET) scan or magnetic resonance imaging (MRI). This is something that Dr Fenton and Dr Pedersen are currently working on. The ability to visualise and identify TLS would also provide opportunities to monitor the effectiveness of new treatments for SLE, as any changes in TLS could be reviewed using PET or MRI imaging.
The scientists explain that in vivo imaging of TLS could be expanded to other diseases that also cause these immune cell aggregates to be formed. This is something that is still relatively unexplored; therefore, their future work will also involve the identification of TLS in other autoimmune diseases and body tissues.
What do we know about in vivo imaging of TLS in autoimmune conditions so far?
The development of TLS has been observed in other autoimmune diseases like Multiple Sclerosis, Diabetes mellitus and Sjøgren’s Syndrome. However, little is done on in vivo imaging of these structures. Radiotracer-labelled immune cells and specific or unspecific tracers have been tested in mice or used in clinical trials to detect inflammation in general. In cancer research, imaging techniques to visualise immune responses and measure mechanism of action during immune-checkpoint blockade treatment are increasing. These new tracers could be exploited in an autoimmune setting and with regard to detection of TLS. Still, improvement of imaging modalities in different disease settings requires additional validation by tissue collections and analyses to confirm the presence of tracer target.
References
- Dorraji, S. E., Kanapathippillai, P., Hovd, A. K., et al. (2020). Kidney Tertiary Lymphoid Structures in Lupus Nephritis Develop into Large Interconnected Networks and Resemble Lymph Nodes in Gene Signature. The American Journal of Pathology, 190(11), 2203–2225. Available at: https://doi.org/10.1016/j.ajpath.2020.07.015
10.26904/RF-135-1217631576
Research Objectives
Dr Fenton and Dr Pedersen study disease progression of systemic lupus erythematosus (SLE).
Funding
- Northern Norway Regional
Health Authority - UiT The Arctic University of Norway
Collaborators
The research team would like to thank all collaborators for their contribution.
Bio

Dr Kristin Fenton is a Professor in immunology/molecular biology and works at the Department of Medical Biology at The Arctic University of Norway. She has worked more than two decades in the field of systemic lupus erythematosus. Her research focus is in the field of autoimmunity with special interest in the development of tertiary lymphoid structures and in vivo imaging.

Dr Hege Lynum Pedersen works as a research scientist and holds a PhD from the Department of Medical Biology at The Arctic University of Norway. She was a Fulbright Fellow at the National Institutes of Health in 2018-2019. Her research interests are in the field of systemic lupus erythematosus with a special interest of lupus nephritis, biomarkers and therapy.
Contact
Faculty of Health Sciences
UiT The Arctic University of Norway
9037 Tromsø, Norway

W: https://en.uit.no/forskning/forskningsgrupper/gruppe?p_document_id=368240
W: https://uit.no/ansatte/person?p_document_id=41969
W: https://uit.no/ansatte/person?p_document_id=43492
Creative Commons Licence
(CC BY-NC-ND 4.0) This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. Creative Commons License
What does this mean?
Share: You can copy and redistribute the material in any medium or format


Low-cost nanoparticles for use across diverse industries




IIED: A global leader in sustainable development

AICR: Committed to cancer research in prevention and survival

