- Rheumatoid arthritis is a disease of the joints that affects half a million people in the UK.
- When the condition progresses it can lead to permanent damage of the joints.
- Early identification of individuals at risk can help prevent the disease’s detrimental consequences.
- Dr Christopher Rooney, Leeds Teaching Hospitals Trust, UK, investigates the gut microbiome of people at risk of rheumatoid arthritis. His findings could help pave the way to early disease treatment.
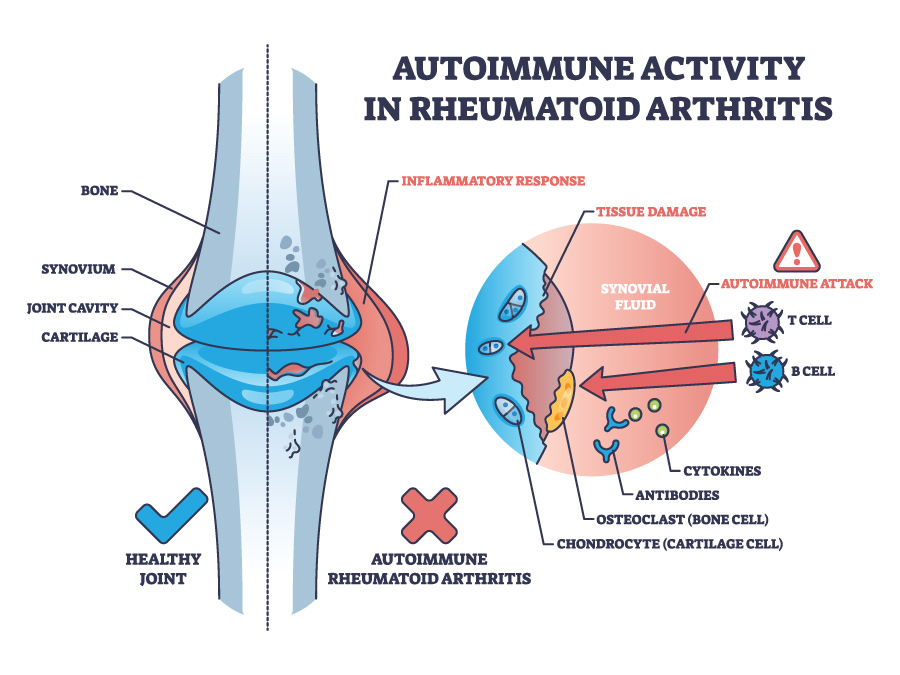
Rheumatoid arthritis (RA) is a disease of the joints that can worsen over time and cause permanent damage to the body. The joints affected are usually those of the hands, feet, and wrists. In some people RA can also affect other parts of the body, or cause more general symptoms such as fatigue. RA is an autoimmune disease, which means that the body’s defence system, which normally fights infection, at some point starts attacking its own joint tissues by producing antibodies against them (initiation of autoimmunity). This causes them to swell, become painful and stiff.

The cause of RA is not entirely clear, but one possible explanation could be given by the mucosal origin hypothesis. This is a theory proposing that inflammation of the body’s cavity lining (mucosa), including the inside of the mouth and the bowel, can trigger a more generalised reaction of the immune system, causing it to produce self-harming proteins such as anticitrullinated protein antibodies (ACPAs) that can attack and damage joint tissues.
The role of the gut microbiome
The gut microbiome, which is the sum of all microorganisms that live on the lining of our bowels, plays an essential role in the body’s health through a number of mechanisms described as host–microbiome interaction.
An imbalance in its community populations (also known as microbial dysbiosis) can lead to diseases of the gut but also of more distant parts of the body. It has been found that people with RA, or at risk of developing RA, are more likely to also have an imbalance of the gut bacterial populations when compared with healthy individuals, including the increase of certain species populations such as particular strains of the Prevotella bacteria.
Gut microbiome imbalance is associated with an increased risk for a number of diseases, including rheumatoid arthritis.
There is currently no cure for rheumatoid arthritis but there are medications that can control the disease and help prevent permanent damage. For these medications to work, the condition must be detected early.
Dr Christopher Rooney (NIHR Academic Clinical Lecturer at the University of Leeds and Leeds Teaching Hospitals NHS Trust) has been researching the microbiome changes in the gut of people at risk of RA with the aim of early recognition and treatment of the affected individuals.
Checking for microbial differences
To study gut changes in people at risk of RA, Dr Rooney and his colleagues recruited participants that formed three groups: 124 individuals at risk of developing RA, seven people newly diagnosed with RA, and 22 healthy people for comparison. The participants at risk of RA were identified by measuring the levels of ACPAs in their blood in addition to having new pains in the joints with no particular findings on examination.

All participants also underwent scans and other blood tests which, in combination with their symptoms, helped to create an RA risk severity score that would be correlated to the findings from their gut microorganisms. Stool samples were also collected, and participants reported any new symptoms via a research helpline. If they had symptoms of RA, they would be reviewed in person to confirm the development of disease. The research team also extracted the genetic material from the microorganisms of the stool samples and identified the different species and strains present in each sample as well as their numbers.
A shift of populations
Of the 124 at-risk participants, 30 eventually developed the disease (progressors). These progressors were found to have significant differences in their gut microbiome populations compared to the individuals who did not develop RA. More specifically, they were found to host larger populations of Prevotella bacteria. One specific strain in particular, Prevotella copri ASV2058, was found in larger numbers in the stool samples of at-risk participants compared to healthy individuals.
The aim is the early identification of people at risk of RA and the prevention of permanent joint damage.
Specific strains of Prevotella were associated with different risk factors for RA. In general, individuals at risk of RA were found to have a less diverse gut microbiome, enriched in certain types of bacteria, including the Prevotella species. It was also found that progressors presented with changes of their gut microbiome, signalling an imbalance of their microbial populations about ten months before the development of disease.
Findings
The study confirmed that individuals at risk of RA have a distinctive gut microbial makeup. The Prevotella abundance in the gut microbiome was found to be associated with high risk of RA development and increased levels of ACPAs. The results also illustrate that there is a late change in the gut microbiome population that leads to a microbial imbalance, associated with RA progression. The controversial differences in the population sizes of the different Prevotella Copri strains suggest that different strains of the species could play diverse roles in RA development, and that the ASV2058 strain could perhaps be used as an additional risk factor for RA.

The gut microbiome is a dynamic environment that can reflect a person’s health. Microbiome imbalance is associated with an increased risk for a number of diseases, including rheumatoid arthritis. The findings of the study are adding to our increasing understanding of this fine balance. Importantly, it could contribute to an early non-pharmacological treatment options and reduce permanent joint damage.
How will further knowledge of the gut microbiome changes help develop preventative RA treatments? What type of studies would be required?
We would like to know how the changes in gut bacteria relate to levels of inflammation in the blood. We could then combine that knowledge into a clinical trial to see how interventions such as diet or other specialised supplements affect both the bacteria and level of inflammation and assess further treatment options.
How can the antibodies, which are part of the immune system, have a direct effect on the microbiome? Is this interaction working both directions?
Yes, there is a delicate dialogue that exists in the gut, between bacteria and the immune system. The gut microbiome is essential for ‘teaching’ the immune system when to respond and when not to respond to certain bacteria. This communication allows the immune system to identify friend from foe. The problem occurs when that conversation breaks down, which leads to local and then body-wide inflammation.










