- Designing an optimal vaccine launch remains a challenge.
- So far, no significant study has attempted to unravel the critical steps towards an optimal vaccine launch.
- Dr Baudouin Standaert from the University Hasselt in Belgium and colleagues have studied the country’s rotavirus vaccine programme for 15 years.
- Their study, the RotaBIS, has led to the design of a four-step approach that could change the future of vaccine launches for the better.
Identifying an appropriate vaccine launch for an infectious disease is crucial for its success. Typically, there are two approaches to launch a vaccine in the market when it is ready. The first approach involves ensuring that the vaccine enters the market on the same day it is approved. The second approach, however, involves a detailed strategy prior to releasing the vaccine to the market, and considers the maximum number of people affected and the long-term socio-economic success of the intervention.
Dr Baudouin Standaert of University Hasselt, Belgium and colleagues strongly believe that when introducing a new vaccine, the second option could lead to better herd immunity in the short term, leading to fewer economic losses long term. Herd immunity or ‘population immunity’ is the indirect protection when a population is immune. Spanning over 15 years, the researchers have conducted a detailed study around the launch of the rotavirus vaccine in a high-income country – Belgium, in this case – defining its flaws and proposing a better launch strategy that may be applicable for future vaccines.
The rotavirus vaccine case in Belgium
Defining an ideal vaccine launch strategy is not an easy task. When a new vaccine enters the market, exploring different launching strategies would be highly unethical towards the people enrolled in such a vaccination study since this could lead to some people intentionally receiving a poor vaccination programme. Ultimately, the best way to describe an ideal vaccine launch is to examine an ongoing vaccination project – which is exactly what Standaert and colleagues did over a 15-year period.
RotaBIS is the first-ever study aiming to uncover the ideal scenario for launching a new vaccine.
Rotavirus is a very contagious virus targeting young children (0- to 5-year-olds). It causes diarrhoea, and the vaccination must be given at a very young age to be safe and effective (6–8 months). One of the first countries that has launched a vaccination programme against the rotavirus was Belgium; the country introduced rotavirus vaccination in 2006. In 2007, Standaert and colleagues designed the RotaBIS (Rotavirus Vaccine Belgium Impact Study), the first-ever study aiming to uncover the ideal scenario for launching a new vaccine in a country, which ran for 15 years. The only vaccination evaluations done at that time were models calculating the cost-effectiveness of the vaccine, led on a company level in 2006.
During the initial phase of their investigation, the researchers collected data on an annual basis from 11 hospitals across Belgium to measure the impact of the vaccination on hospitalisations. Overall, the results from the RotaBIS revealed that there was not a strong response to the vaccine in the first years, while the impact curve on hospitalisations (showing the overall impact on the target population) reached a ‘plateau’, ie, maximised after three years. But surprisingly, new small biennial disease peaks appeared in the target group (2- to 5-year-olds) around 9 years after the vaccination had been initiated.
The RotaBIS concluded that the vaccine programme achieved only a 70% drop in the positive rotavirus tests for hospitalisation after five years due to a poorly timed start date of the vaccination programme. The annual peak season of the infection in Belgium is known to be from February to the end of March, indicating that the vaccinations should have been introduced 8–10 months before then. If timely introduction of the vaccine had taken place, the researchers estimate there would have been a 90% drop in positive tests, and the herd effect would have protected the unvaccinated older children from infections and disease over a long period of time.
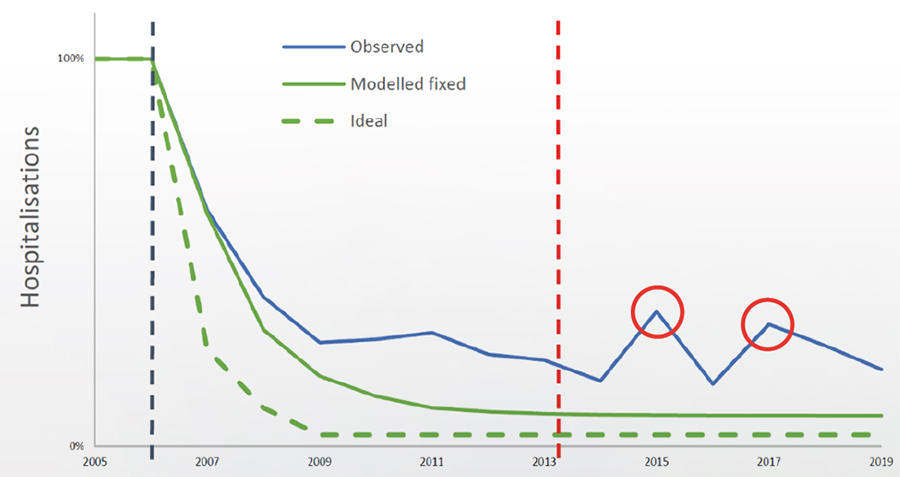
The analysis also depicted that the target for infections shifted to older children within the target group, due to the failure to achieve a high herd immunity at the start, causing the appearance of the small peaks later on and thus the hospitalisation rate to rise (figure 1).
The steps towards a good vaccine launch
Is it possible to achieve an ideal scenario? Notably, countries where the vaccine launch aligned to the model predicted by Standaert and colleagues, such as the UK and Finland, obtained much better results compared to Belgium. The effect of an ideal scenario is direct; it has the potential to dramatically decrease the hospitalisations of children and act as a catalyst for the economic assessment of the vaccine campaign in the long term. The researchers emphasise that for an ideal vaccine launch, the cost–impact result needs to be the same as the cost-effectiveness, indicating that the results depend on the overall performance of the vaccination strategy, having no consequences anymore on the spread of infection in unvaccinated people over time.
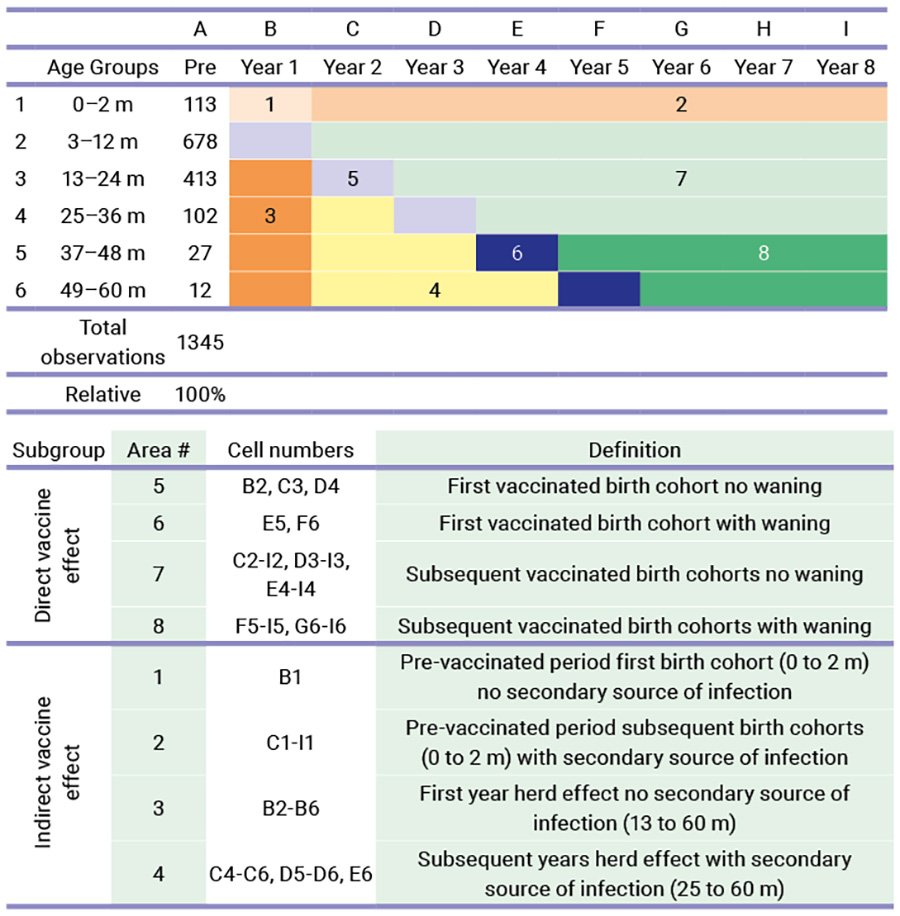
After careful analysis of the RotaBIS case in Belgium, the researchers concluded that the ideal vaccine launch consists of four distinguished action points, of which the first two should occur before the launch. The first action point involves an in-depth understanding of the infection and the disease epidemiology, including the infection speed and the factors it influences (seasonality, gender, or ages targeted, primary and secondary sources of infection, and the cost data). The second action point involves modelling the epidemiology, taking into account the findings from the previous step. The modelling construct will include the vaccine launch and encompasses a period called the vaccine uptake period. The duration of that period is until the infection spread reaches its new infection equilibrium in the target group, with the vaccination routinely implemented. This typically happens when the target group to be vaccinated has been fully covered. In the model, any other factors that could influence the vaccination should be investigated, such as the effectiveness or the waning of the vaccine (direct forces) and other factors, such as the herd effect and the appearance of secondary sources of infection (indirect forces).
The third action point is also a modelling process. Here, the long-term effect of the vaccination in the post-uptake period is processed given the dynamicity of the infection spread under control (or not) of the vaccine. That period comes late in the process after initiating the vaccination programme. In the case of RotaBIS it started 8 years after the beginning of the vaccination when the new smaller peaks of disease occurred every two years (biennial peaks). The fourth and final action point is to continuously monitor the effect of the vaccine. This is achieved by collecting the same data as at initiation of the study. A comparison between the models initiated and the real-life data can be made, to help us understand and find explanations for potential deviations observed. The fourth action point is most critical for identifying an ideal vaccine launch as a lesson learned for others who will initiate this vaccination.
Designing wiser vaccine launches
Standaert highlights that in the future, before entering the market, the disease and the vaccines need to be studied thoroughly, and the decision-makers should consider the four-action-point approach for initiating an optimal vaccine launch. Vaccines designed for known infections in a known population could vastly benefit from this approach. Newer vaccines could also benefit, such as vaccines against the retroviral syncytium virus (RSV).
Vaccines designed for known infections in a known population could vastly benefit from this approach, with major clinical and socio-economic benefits to obtain the best return on investment.
Vaccines against many other known and well-studied diseases, such as those against pneumococcal infection and human papillomavirus infection, could follow this multi-step approach. Ultimately, with this knowledge, we could walk towards carefully planned vaccine launches with great benefits for both the health condition of a country’s population and its socio‑economic development.
Do you expect differences in the infection profile between the rotavirus vaccine and vaccines for other known infections?
Absolutely. Every infection is different. It can’t be completely the same, each has its own characteristics of contagion, preferred target group, dominance patterns, sources of infection, and temporal spread. We need to study this in depth, well in advance before any new vaccine is launched against a particular infection. It helps define the best vaccine launch scenarios with short-term (uptake period) and long-term (post-uptake period) effects.
Would you expect practical difficulties when implementing the four-action-point approach for a vaccine strategy against retroviral syncytium virus (RSV)?
Many different interventional approaches have been developed that will soon come on the market for this disease. This complicates finding the right combination of interventions to control the infection in the shortest period of time (objective for the authorities). The problem of RSV infection control is more complex than for rotavirus, exacerbated by the fact that the disease is also very much present in different age groups (the very young and the ageing population). But the four-action-point approach, with the first two steps well developed, is a must for a clear prevention strategy for short-term gain to long-term benefit. It is an interesting but challenging problem in which constrained optimisation models could be very helpful.
What would be the benefits of governments adopting the proposed ideal vaccine launch?
Nowadays, new vaccines don’t have the unique focus of reducing mortality as in the past. Today, the benefits of vaccination must be seen as much broader: it’s not only a gain for the individual being vaccinated, but also for the healthcare system and society as a whole, with a reduction in hospitalisations, improvement in quality of care, reduced seasonal burden of infection for primary healthcare, gain for employers and employees due to a reduction in work absenteeism, fiscal gain for the authorities to spend less on costly healthcare and paying compensations for sick leave, and working parents who don’t have to stay home and care for a sick child as often. All these different elements have been demonstrated and reported in separate publications. They are quite substantial for the socio-economic gains of society as proven by the COVID-19 vaccine.
How do you envision the four-action points approach being implemented in low‑income countries?
The four-action-points approach is a scenario developed in high-income countries. Why? Because the launch of rotavirus vaccination in high-income countries was not easy, even today. The main driver for vaccination, normally the high disease mortality, was not so prominently present in high-income countries for rotavirus disease. Introducing a new vaccine at the population level is always a big investment in prevention, and the authorities need to understand the full gain of their return on investment. We therefore had to be very precise in the working and the benefits generated by the vaccine – because with good prevention nothing should happen, and that is a very difficult story to sell unless you can clearly demonstrate and illustrate what was before and what comes after the vaccination. The focus was to find the best vaccine launch by which you can demonstrate when and where the greatest benefit of the vaccine will appear under ideal circumstances.
In low-income countries, the priority setting for vaccination differs greatly from high-income countries. Here, the primary focus on reducing mortality drives the vaccination strategy. However, the four-action-point approach, based on the experience in high-income countries, could also help to obtain a better-focused approach in low-income countries if there is, for instance, seasonality in the infection spread which would recommend a clear starting date of the vaccination programme. It’s therefore always critical to understand the disease in all its aspects in any specific environment before the vaccine is newly introduced. However, facilities to collect information about the disease and its spread in the community do not always exist or may be cumbersome to develop and assemble, in part because the priority setting is so different (mortality reduction). Therefore, the focus in low-income countries will often be to maximise vaccine coverage of the target group.
What reasons do you attribute to the past lack of a similar study investigating the right path towards a suitable vaccine strategy?
Pressure among the producers of new vaccines to be the first on the shelf determines the price of the vaccine. Also, those rapid processes don’t consider that introducing a vaccine in a population may cause heavy disturbances regarding health benefits and consequences. The Belgian case of rotavirus introduction is a good illustration of a too-rapid introduction of the vaccine without knowing the long-term consequences. People considered the 70% reduction in hospitalisations a great success; however, they could have obtained better results if they thought about an ideal launch scenario. Now, Belgium is exposed to biennial infection peaks that are difficult to attenuate because the vaccine has no direct impact on those secondary sources of infections causing those peaks. In that respect, the RotaBIS was a great initiative as it helped us discover those findings. If we had done the same study in the UK or Finland, we wouldn’t have seen the short- and long-term consequences of a non-ideal vaccine launch.











