Replicating human tissues in vitro requires cultivating cells under controlled conditions. Hydrogel-based three-dimensional (3D) cell cultures can realistically mimic various aspects of both healthy and diseased human tissues. However, the complexity of these models has made their adaptation to high-throughput applications a significant challenge.
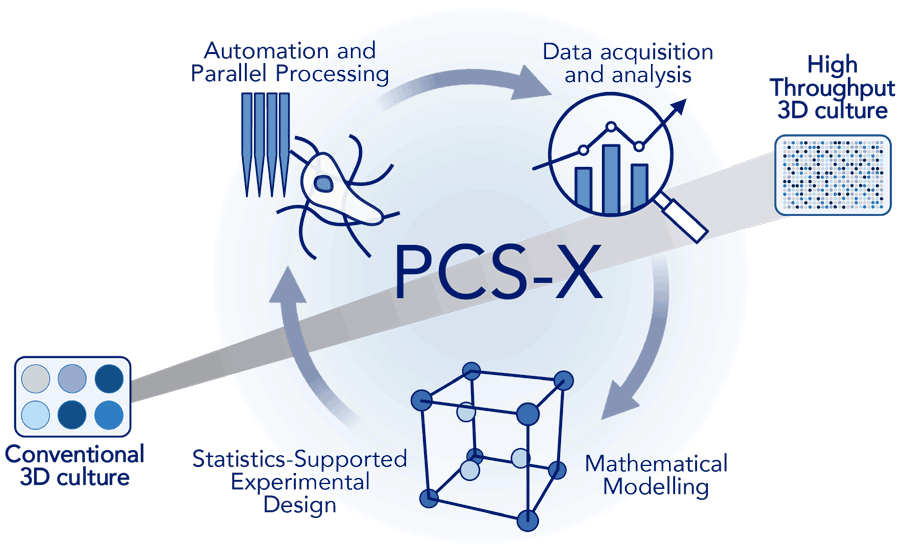
To address this, Professor Carsten Werner’s team at the Leibniz Institute of Polymer Research Dresden pioneered Precision Culture Scaling (PCS-X). By integrating statistical experiment planning, automation, advanced image analysis, and mathematical modelling, PCS-X streamlines the customisation of hydrogel-based 3D cell cultures, making true high-throughput research applications a reality.
Three-dimensional (3D) cell cultures have become indispensable tools in biomedical research, providing platforms that closely model human tissue structure and function in vitro. They significantly enhance our understanding of diseases, accelerate the discovery and testing of novel therapeutics, and support the development of personalised treatments.

Hydrogels: A Leap Forward in In Vitro Tissue Modelling
Expanding the advantages of 3D cell cultures, hydrogels – 3D networks of hydrophilic polymers – have emerged as powerful tools for in vitro tissue modelling. By recapitulating the structural and biochemical properties of the extracellular matrix, hydrogels enable cells to grow and interact, approximating in vivo conditions.
Professor Carsten Werner and his team at the Leibniz Institute of Polymer Research Dresden have conducted seminal work in this field. They explored how specific hydrogel properties and the combination of different cell types influence tissue formation. Their work has propelled the use of hydrogels in biomedical research and paved the way for more complex, predictive in vitro models.
Tackling the Challenge of Scaling
Despite the promise of hydrogel-based 3D cultures, scaling in vitro models for high-throughput applications – where numerous samples are tested simultaneously – remains a significant challenge. Their inherent complexity, smaller culture wells and nutrient diffusion limitations all hinder the adaptation of low-throughput protocols to large-scale setups.

Achieving truly high-throughput 3D hydrogel-based models therefore necessitates careful optimisation of hydrogel composition, processing methods, cell seeding density, culture nutrients, and analytical techniques – all while preserving consistency and reliability. Recognising this need, Professor Werner and his team developed a coordinated method that makes hydrogel models suitable for both high-throughput studies and accurate tissue physiology modelling.
By recapitulating the structural and biochemical properties of the extracellular matrix, hydrogels enable cells to grow and interact in three dimensions.
Precision Culture Scaling (PCS-X): A Game-Changer in High-Throughput Applications
This method, called Precision Culture Scaling (PCS-X), combines several key techniques to address the complexities of hydrogel-based 3D cell cultures in a streamlined and sustainable manner. The core components of PCS-X include:
• Statistics-Supported Experimental Design: Design of Experiments methodologies are used for effective planning and optimisation of experiments. By varying multiple parameters at once, they enable the identification of the most influential factors and optimal conditions for cell growth and tissue formation.
• Automation and Parallel Processing: Automated liquid handling systems and parallel processing techniques facilitate the production of numerous 3D culture samples at once, boosting throughput and minimising variability between samples.
• Data Acquisition and Analysis: Advanced imaging technologies and sophisticated image analysis tools enable real-time tracking and quantitative evaluation of cells within 3D cultures. This provides in-depth understanding of cell behaviour, morphology, and interactions.
• Mathematical Modelling: Mathematical models applied to experimental data can pinpoint optimal conditions for cell growth. This predictive modelling not only improves the efficiency of experimental design but also speeds up the optimisation process.

PCS-X’s potential was exemplified by customising hydrogel-based high-throughput 3D vasculogenesis models involving both highly proliferative umbilical cord-derived endothelial cells and fully differentiated ocular endothelial cells.
PCS-X streamlines the creation of high-throughput 3D culture models, thus benefitting multiple areas of research – particularly oncology, regenerative therapies, and personalised medicine.
These models were employed to simultaneously study the effects of multiple vasculogenesis inhibitors across a range of concentrations – showcasing PCS-X’s potential for high-throughput drug screening and complex biological research.
PCS-X: Pioneering Ethical and Personalised Medicine
PCS-X can transform the production of high-throughput, physiologically relevant 3D cell cultures by offering a more precise, cost-effective, and ethically responsible method for simulating human tissue behaviour in vitro. Its flexible approach to hydrogel composition allows researchers to create tissue models that closely mimic the mechanical, structural, and biochemical characteristics of human tissues and organs. Incorporating patient-specific cells captures individual genetic and biological nuances, enabling more accurate drug testing, targeted treatment regimens, and advancements in regenerative medicine. Moreover, PCS-X streamlines large-scale drug screening by reducing both costs and development timelines, with broad potential applications in oncology, regenerative therapies, and personalised medicine.
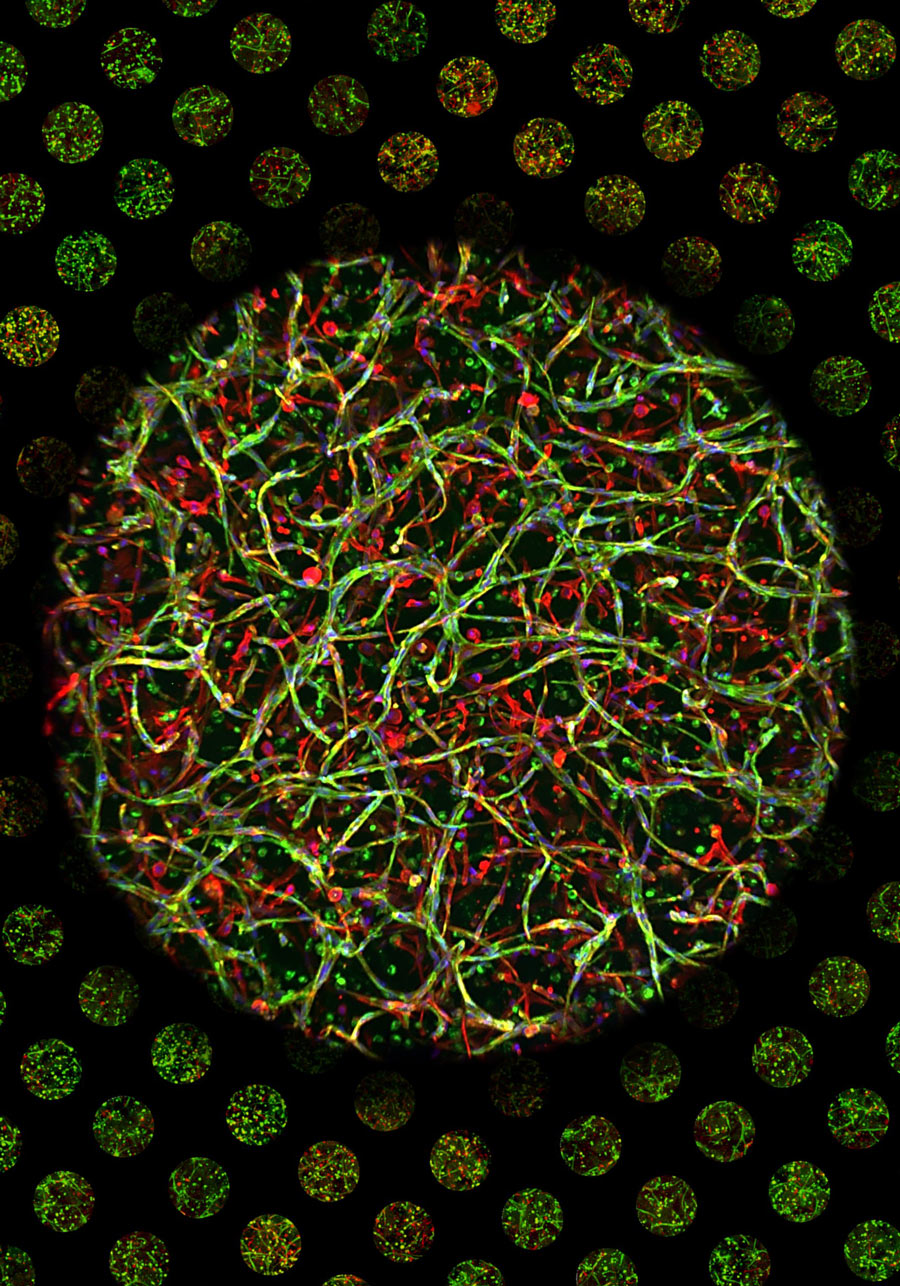
Copyright IPF, Maximilian Fusenig, Nicolas Robert Dennison
As medical science advances, technologies like PCS-X will be pivotal in bridging the gap between in vitro studies and clinical applications, providing scalable, human-relevant testing environments that reduce reliance on animal models and deepen our understanding of disease complexity and therapeutic efficacy. Ultimately, by enhancing data quality and accelerating research, PCS-X paves the way toward improved patient outcomes.

How will the wide adoption of PCS-X change high-throughput drug discovery and tissue engineering, and what fields in the biological and medical sciences will benefit most from the use of this methodology?
The wide adoption of PCS-X will transform high-throughput drug discovery and tissue engineering by enabling efficient, scalable, and accurate 3D cell culture models that closely mimic human tissues. PCS-X streamlines the creation and optimisation of complex tissue models, facilitating the rapid screening of drug candidates in physiologically relevant environments. This methodology will significantly benefit fields such as cancer research, regenerative therapies, and personalised medicine, where understanding complex cell interactions and responses is crucial. By reducing reliance on animal models and enhancing predictive accuracy, PCS-X fosters more ethical and effective approaches in biomedical sciences.











